Haz 06, 2019
Alverno Laboratories goes digital with Philips to innovate pathology services
Large Midwest laboratory selects Philips IntelliSite Pathology Solution to further enhance efficiencies and quality of diagnostics for histology cases
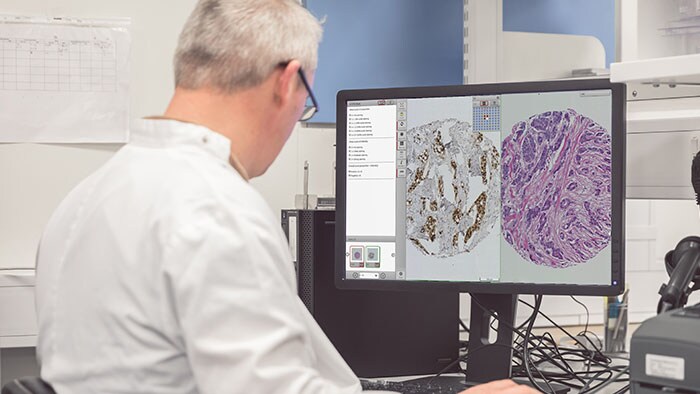
Amsterdam, The Netherlands and Hammond, Ind. – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, and Alverno Laboratories, a provider of high-quality diagnostic testing services, today announced that Alverno will fully implement digital pathology in its clinical laboratories. The laboratory network will use Philips IntelliSite Pathology Solution to assess and diagnose all clinical histology cases digitally, instead of using a microscope, with the aim of improving laboratory efficiency, quality, and patient safety. Pathology plays a critical role in disease detection, particularly with cancer diagnosis. Suspicious tissue samples are investigated to determine if the tissue is malignant and consequently guides treatment decisions. As one of the largest pathology laboratories in the Midwest, Alverno manages 33 hospital laboratories and provides laboratory services to two freestanding emergency departments and thousands of physician offices and other clients. Alverno consults on more than 142,000 histological cases each year, which translates to more than 1,100,000 slides of human tissue. Currently, each of these slides needs to be prepared, analyzed through a microscope, diagnosed, reported and archived. Digitizing these images will ease collaboration across sites and help reduce costs.
Digital pathology enables enhanced cooperation and access to sub-specialists, helping us improve turnaround times and ultimately advancing our goal of saving lives.
Sam Terese
CEO of Alverno Laboratories
“Alverno is committed to ensuring our patients and clinical colleagues receive the fastest, most effective and best-informed diagnoses possible by employing the latest technology innovations,” said Sam Terese, CEO of Alverno Laboratories. “Digital pathology enables enhanced cooperation and access to sub-specialists, helping us improve turnaround times and ultimately advancing our goal of saving lives.” “The digitalization of pathology provides an opportunity to radically change the sector and remove daily obstacles that hinder pathologists via traditional microscopy,” said Marlon Thompson, General Manager of Philips Digital & Computational Pathology. “With digital pathology and the application of adaptive intelligence, we aim to help pathologists increase collaboration, boost efficiency and enable more accurate and precise diagnoses.” Philips IntelliSite Pathology Solution is an automated digital pathology image creation, viewing, and management system comprising the Ultra Fast Scanner, the Image Management System and Display for clinical IVD use. This solution contains advanced software tools to manage the scanning, storage, presentation, reviewing, and sharing of images. By supporting the transition to digital workflows, Philips seeks to help pathology laboratories simplify access to histopathology information and implement more efficient workflows. The Philips IntelliSite Pathology Solution is the first digital pathology solution marketed for primary diagnostic use in the U.S, for all FFPE surgical pathology slides, including H&E, special stains and immunohistochemistry. To learn more about Philips IntelliSite Pathology Solution, visit this website and follow @Philips_Path. To learn more about Alverno Laboratories, visit www.AlvernoLabs.com.
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and enabling better outcomes across the health continuum from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips generated 2018 sales of EUR 18.1 billion and employs approximately 77,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
About Alverno Laboratories
Alverno Laboratories is one of the largest integrated laboratory networks in the United States and owns and operates over 30 hospital laboratories and a central laboratory performing both clinical and anatomic pathology. Alverno's innovative, state-of-the-art laboratory offers cost effective testing and personalized customer service, with advanced technology and testing in Precision Medicine/Next-Gen Sequencing, toxicology and other speciality testing. Alverno's on-site pathologists are part of the pathology practice of Pathology Consultants, Inc. Alverno is located in Hammond, IN. For more information, call 1-800-937-5521 or visit http://alvernolabs.com.
Topics
Contacts

Joost Maltha
Philips Global Press Office Tel: +31 6 10 55 8116
You are about to visit a Philips global content page
ContinueSierra Breeden
Alverno Laboratories Tel.: 219-836-2458 Cell: 815-791-9467
Media assets
Press releases
Get our press releases by e-mail
You are about to visit a Philips global content page
Continue